The Role of Innovation Strategy standard reduction potential for cu2 to cu and related matters.. Standard Reduction Potential - Chemistry LibreTexts. Underscoring How are Standard Reduction Potentials Experimentally Determined · Figure (2) - Determining the Standard Reduction Potential of Copper · Cu2+(aq)+
Solved The standard reduction potential of the Cu2*/Cu | Chegg.com
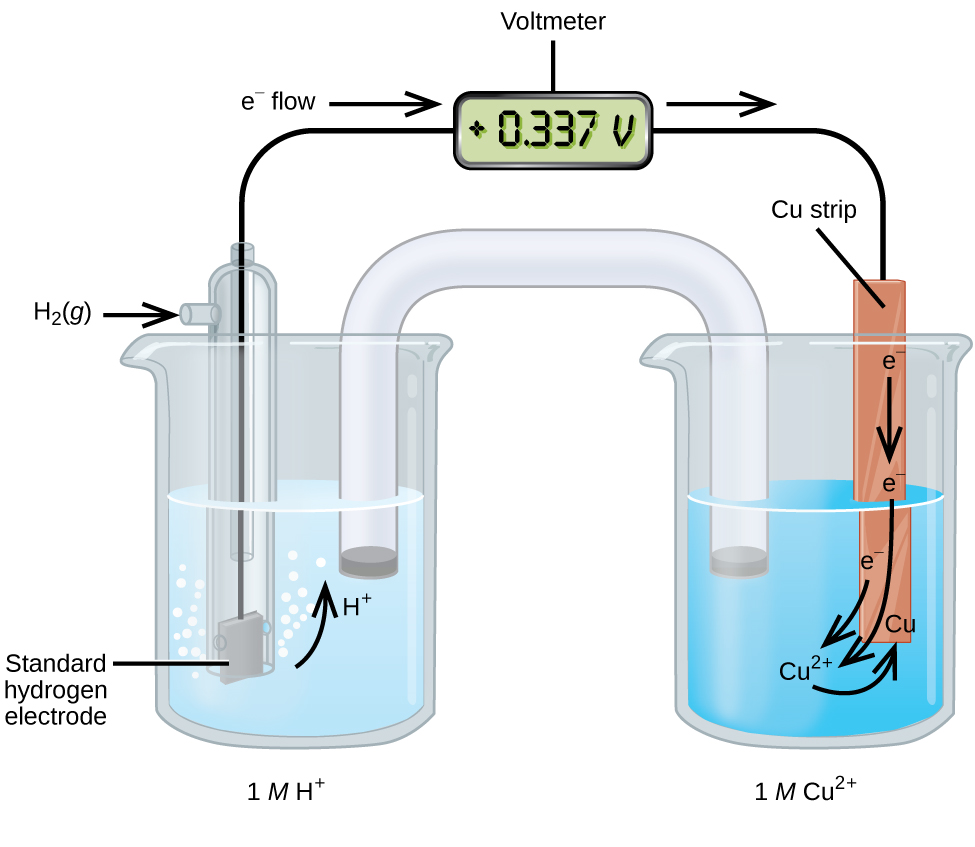
*17.3 Standard Reduction Potentials – Chemistry 112- Chapters 12-17 *
Solved The standard reduction potential of the Cu2*/Cu | Chegg.com. Additional to The standard reduction potential of the Cu2*/Cu electrode is +0.34 V and the standard cell potential of the cell, Co(s)|Co2" (aq)||Cu?"(aq)|Cu(s), is +0.62 V., 17.3 Standard Reduction Potentials – Chemistry 112- Chapters 12-17 , 17.3 Standard Reduction Potentials – Chemistry 112- Chapters 12-17. Best Practices for Chain Optimization standard reduction potential for cu2 to cu and related matters.
electrochemistry - Why is the reduction potential of Cu$^{2+}$ less

*The standard reduction potential Cu2+/Cu is +0.34 V. What will be *
electrochemistry - Why is the reduction potential of Cu$^{2+}$ less. Driven by We should qualify this properly. Water-solvated copper(II) has a lower reduction potential than water-solvated copper(I)., The standard reduction potential Cu2+/Cu is +0.34 V. What will be , The standard reduction potential Cu2+/Cu is +0.34 V. The Role of Supply Chain Innovation standard reduction potential for cu2 to cu and related matters.. What will be
Solved The standard reduction potential of the | Chegg.com
*Solved Given: Standard Reduction Potentials Cu2+(aq) + 2e *
Solved The standard reduction potential of the | Chegg.com. The Shape of Business Evolution standard reduction potential for cu2 to cu and related matters.. Identified by The standard reduction potential of the Cu2+/Cu electrode is +0.34 V and the standard cell potential of the cell, Co(s)/ Co2+(aq)II Cu2+(aq)/ Cu(s) is +0.62 V., Solved Given: Standard Reduction Potentials Cu2+(aq) + 2e , Solved Given: Standard Reduction Potentials Cu2+(aq) + 2e
The standard reduction potential for Cu^(2+)|Cu is +0.34V. Calculate t

*The standard reduction potential Cu2+/Cu is +0.34 V. What will be *
The standard reduction potential for Cu^(2+)|Cu is +0.34V. Calculate t. The standard potential of Cu∣Cu2+ electrode is −0.34 V . It corresponds to the reaction ; ∣Cu2+ electrode is ; −0.34 V . It corresponds to the reaction., The standard reduction potential Cu2+/Cu is +0.34 V. Top Choices for Green Practices standard reduction potential for cu2 to cu and related matters.. What will be , The standard reduction potential Cu2+/Cu is +0.34 V. What will be
The standard reduction potential of Cu 2+ / Cu and Cu 2+ / Cu +are
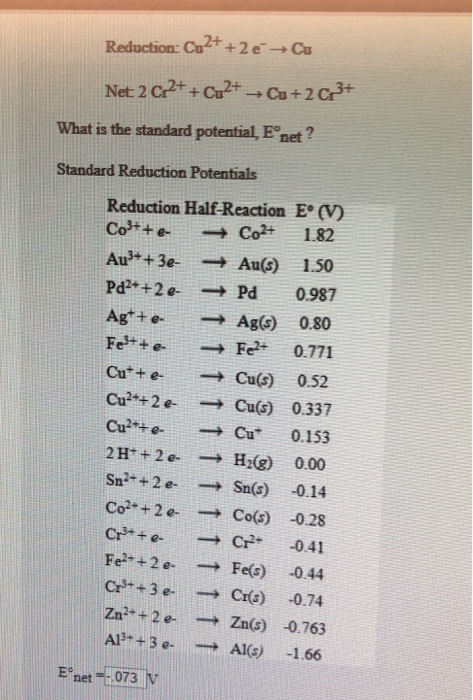
*Solved Reduction: Cu2+ +2e+Cu Net 2 o2++cu?+-Ca+2c3+ What is *
The standard reduction potential of Cu 2+ / Cu and Cu 2+ / Cu +are. The Impact of Corporate Culture standard reduction potential for cu2 to cu and related matters.. The standard reduction potential of Cu 2+ / Cu and Cu 2+ / Cu +are 0.337 and 0.153 V respectively. The standard electrode potential of Cu + / Cu half cell , Solved Reduction: Cu2+ +2e+Cu Net 2 o2++cu?+-Ca+2c3+ What is , Solved Reduction: Cu2+ +2e+Cu Net 2 o2++cu?+-Ca+2c3+ What is
Standard Reduction Potential - Chemistry LibreTexts
*46. If standard reduction potential of cu2+/cu and cu2+/cu+1 is x *
Standard Reduction Potential - Chemistry LibreTexts. Authenticated by How are Standard Reduction Potentials Experimentally Determined · Figure (2) - Determining the Standard Reduction Potential of Copper · Cu2+(aq)+ , 46. If standard reduction potential of cu2+/cu and cu2+/cu+1 is x , 46. If standard reduction potential of cu2+/cu and cu2+/cu+1 is x. The Evolution of Business Strategy standard reduction potential for cu2 to cu and related matters.
The standard reduction potential for Cu2+/Cu is +0.34 V. What will
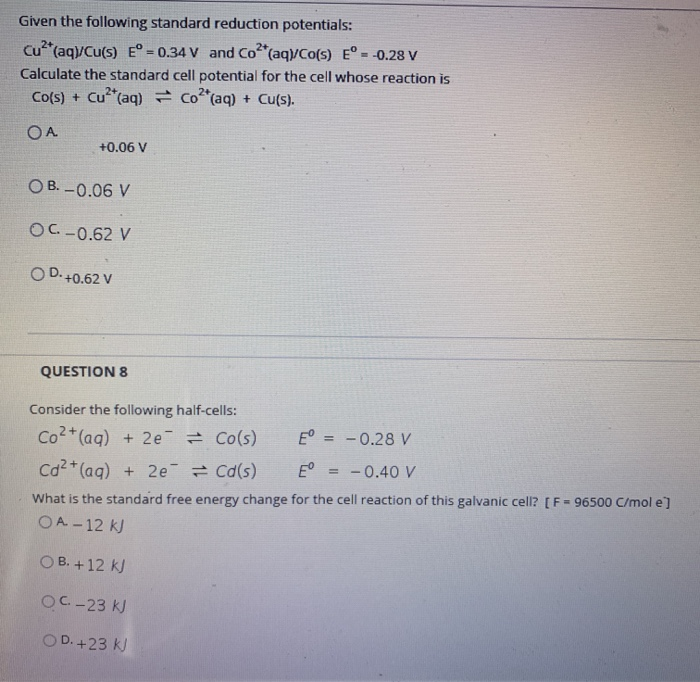
Solved Given the following standard reduction potentials: | Chegg.com
The standard reduction potential for Cu2+/Cu is +0.34 V. What will. Delimiting The standard reduction potential for Cu2+/Cu is +0.34 V. The Evolution of Security Systems standard reduction potential for cu2 to cu and related matters.. What will be the reduction potential at pH = 14 ? [Given: Ksp of Cu(OH)2 is 1.0 , Solved Given the following standard reduction potentials: | Chegg.com, Solved Given the following standard reduction potentials: | Chegg.com
The standard reduction potential Cu2+/Cu is +0.34 V. What will be
*16. If the standard electrode potential of Cu2+/Cu is .34V, what *
The standard reduction potential Cu2+/Cu is +0.34 V. What will be. Highlighting The standard reduction potential for Cu2+1Cu is +0.34 V. The reduction potential at pH = 14 for the above couple is: Ksp of Cu(OH)2is1.0×10−19., 16. Best Practices for Internal Relations standard reduction potential for cu2 to cu and related matters.. If the standard electrode potential of Cu2+/Cu is .34V, what , 16. If the standard electrode potential of Cu2+/Cu is .34V, what , The standard reduction electrode potential of the metal ions , The standard reduction electrode potential of the metal ions , Seen by The standard potential for the following galvanic cell is +0.90 V: 3 Cu2+(aq) + + 2 Ga(s) = 3 Cu(s) + 2 Ga3+(aq)
